6 minute read
MedTech Compliance: How to manage increasing regulation
MedTech compliance: Successfully navigate through regulations. Learn how to optimize resources, streamline data, & implement effective systems.
Table of contents
Increased regulations for EU MDR compliance may result in a resource strain, but they also present an opportunity for MedTech and IVDR (in vitro diagnostic medical. devices regulation) companies that embrace this pivotal moment wisely.
As a result of the European Union Medical Device Regulation (EU MDR) entering into application on May 26th, 2021, the Life Sciences industry has been shaken and transformed.
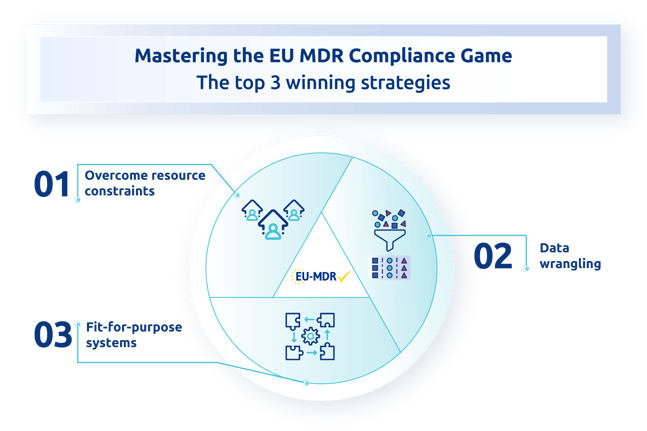
This article will tackle the top 3 key factors that put you on the winning side of this new EU MDR compliance world:
- Overcome resource constraints
- Data wrangling
- Fit-for-purpose systems
MedTech regulations: The reason why
Before jumping into speaking on EU MDR company strategy tips, it is important to understand the key reason why EU MDR and other similar regulations have come into action around the globe.
MedTech compliance regulations aim to protect the end users of medical devices, pharmaceuticals, and healthcare products and services. The main goal is keeping patients and users safe.
To exist in this new highly regulated world, each company must achieve a state of public transparency and continuous product performance monitoring for their finished goods and services on the market.
They must have a full audit trail for all critical product-related data and prove that the health benefits of their product or therapy clearly outweigh the risks and side effects, all while running a successful and competitive business.
All this and also what will follow is not limited to just EU MDR, but can also be applied to other MedTech and Pharmaceutical increasingly stringent regulations and regulators, for example, 21 CFR 820, ISO 13485, JPAL, CDSCO, MHRA, etc.
The 3 key winning factors
#1 key factor: Overcome resource constraints
With added regulations comes an extra burden.
How do we tackle this challenge in today’s world? There really are only three options:
- Working harder;
- Hiring more people;
- Working smarter.
Working harder can lead to burnout, mental health issues, and employee dissatisfaction.
Hiring more people may be a remediation option, but the key subject matter experts (SMEs) needed to manage and evaluate clinical evidence have been quickly snapped up and gone to the highest bidders. The SMEs required to be successful are now either not available on the market or are not cost-effective for a long-term strategy.
Working smarter seems to be the best option. This is a challenge and opportunity at the same time.
Working smarter
Every company, regardless of size, would like:
- A lean resource model;
- SMEs focused on their value-added tasks;
- Minimisation of tedious administrative tasks;
- Highest quality and best-selling products possible.
Working smarter is the way of today, and it is the way of the future. You need to free up time from resources you already have, and this takes transformation.
At a small scale, this looks like using Excel macros, subscribing to expert services, using simple apps to automate a few processes, or maybe it is leveraging trending AI platforms like ChatGPT to collect public information quickly or even act as a work aid.
At an enterprise level, there are obviously more complexities to consider. A few key items may include:
- Digitisation of processes and records to bypass hinderances of paper processes;
- Interoperability of systems to avoid overhead related with siloed data sets (ie. systems “talk to each other”);
- Collaborative tools instead of desktop thinking;
- Workflow automation;
- Structured authoring tools to replace standard word processor software;
- Dash-boarding software to allow continuous monitoring without repetitive manual tasks.
Resource constraints cannot be addressed without investing in connected systems and intelligent tools that do the heavy lifting for you. The dashboards, AI systems, and workflow automations are not possible without having a high-quality set of data as the bedrock.
Working smarter and properly addressing resource constraints are much more effective if we start at the foundation: Data.
#2 key factor: Data wrangling
Data wrangling, also known as data cleaning, data remediation, or data munging, encompasses a range of procedures aimed at converting raw data into formats that are easier to utilise.
This process is needed in MedTech and Pharma industries that have to organise and manage billions of data and records.
For example, Datavid has worked with different enterprise companies that have some critical things in common:
- They are on a path of digital transformation – taking legacy processes and making them electronic;
- They are bringing information into a common warehouse to then leverage across the organisation;
- They are consolidating systems into one single choice wherever possible;
- They are moving away from desktop applications in favour of cloud-based tools;
- They are implementing connected tools that drive efficient collaboration;
- They are taking security, data privacy, compliance and future-proofing very seriously.
Today, it is possible to solve challenges such as combining many structured and unstructured data sets into one common data warehouse, or consolidating PLM (Product Lifecycle Management) systems, Excel job aids, Post Market Surveillance (PMS) systems, or clinical data sets into one intelligent and enriched data set.
How can Datavid help you with data wrangling
Datavid has skilled team members, expertise in the MedTech industry and the best solutions to consider. With a product like Datavid Rover, many company data stores can be combined and made intelligent quickly.
End users don’t want a giant data lake though. They need a tool that helps users by enriching the data to make the information more usable and contextualised for the relevant businessperson.
It is useful to have a tool that can give the end user the exact snippet of information that they are looking for, at the moment that they need it. It can also identify key concepts and relationships within the data, so that new insights can be gained more quickly.
For example, if you have many clinical research articles siloed in R&D, Clinical and PMS libraries across your organisation, just flat articles stored as if they’re in network folders, typically in a PDF file type.
With an out-of-the-box (OOTB) product like Rover, these articles can be consolidated, enriched with tagging and Object Character Recognition (OCR), made searchable and accessible through a centralised and friendly user interface, and then further enhanced for contextualisation and data extraction.
Even just 5 years ago, a project to accomplish these things at an enterprise level would have cost millions of dollars and more than 3 years according to some of our customers. That same type of functionality is now available OOTB with a rapid configuration and deployment plan.
This data wrangling exercise leads to huge potential and many pathways to value. It all starts with gathering and organising data, however.
Imagine the insights and workflows that can be created once your product performance data from PMS, Clinical, and Risk Management systems is layered on top of the PLM and R&D medical device data foundation. This aggregated data and enrichment is what can put you on the winning side of EU MDR.
To gain a rich ROI from wrangling your data you have to build crafty systems on top of that brilliant groundwork.
#3 key factor: Fit-for-purpose systems
If your company has an aggregated and enriched data set that can be leveraged for use across the organisation, all those things mentioned above become possible.
In this case, this is what would happen:
- Systems talk to each other, and they can drive a business process.
- What was an email and attachment and meeting type of process, has been transformed into actionable workflows.
- Process handoffs are seamlessly completed in an end-to-end (E2E) software tool that is role-based, with appropriate read/write or reviewer/approver configurations integrated.
- All users start to look at the same single source of truth in a common controlled location.
- The GxP compliance folks are happy because the business process now occurs in a fully validated environment.
- The users are happy because they spend less time trying to make sense of emails and bouncing from meeting to meeting.
- Management is happy because less time wasted means prevention of extra head count and also that the work assignments actually get completed.
It’s a win-win.
One by one, you can start to automate the highest priority workflows and freeing up expensive resources from tedious tasks. So that your employees can do the work they enjoy doing, and what they’re truly hired to do as an SME.
The most important thing for you when trying to tackle EU MDR compliance efficiently and effectively is then structured content authoring with visualisation of data and AI over the top of the entire landscape.
Structured authoring tools allow for collaborative authoring instead of a desktop application like Microsoft Word. The benefits are massive, but largely untapped within the Life Sciences industry because it is a significant technology challenge.
Benefits of MedTech compliance
Imagine using reusable content in a structure authoring tool to drive reductions on the order of 50% for writing documents like Periodic Safety Update Reports (PSURs), Summary of Safety and Clinical Performance (SSCPs), or Clinical Evaluation Reports (CERs).
At an enterprise level, the savings on authoring just these types of documents in an effective structured authoring tool can be upwards of $10m per year.
With structured authoring tools in place, workflows can be automated, monitoring and reporting can be a continuous integrated pipeline, and the foundation is set to add machine learning or AI on top. All things are possible once the fundamentals are in place.
With an OOTB product like Datavid Rover, the foundation can be achieved quickly, effectively, and with these aspects already built into the vision.
No matter if you’re a big enterprise or working at a small to medium business in MedTech or Pharma, this fundamental process to conquering global regulatory compliance challenges can be catered to your size.
Frequently Asked Questions
What are the main compliance regulations that MedTech companies need to follow?
MedTech companies must comply with a range of regulations depending on their operating regions. Key frameworks include the FDA’s 21 CFR Part 11 in the U.S., the EU Medical Device Regulation (MDR), ISO 13485 for quality management, and GDPR for data protection. Staying compliant requires continuously monitoring updates and aligning internal processes with evolving standards.
How can smart data management improve MedTech compliance?
Smart data management enables automated tracking, classification, and retrieval of regulatory data. By using tools like semantic data platforms and knowledge graphs, MedTech firms can ensure traceability, reduce manual errors, and streamline audits. This leads to faster submissions, fewer compliance risks, and improved product lifecycle management.
What challenges do MedTech companies face in maintaining regulatory compliance, and how can they overcome them?
Common challenges include siloed data, lack of real-time oversight, and rapidly changing regulations. Overcoming these requires a unified data strategy that supports interoperability, data lineage, and auditability. Leveraging technologies such as AI-assisted document processing and regulatory knowledge graphs can help MedTech firms stay agile and compliant.